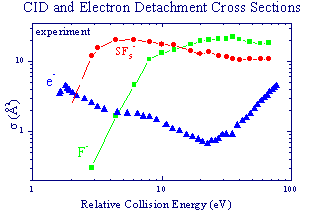
Collisions of SF6- with N2 are obviously
involved in such mixtures when
used as gaseous insulator. Measured cross sections for electron detachment
and collision-induced dissociation (CID) of SF6- can,
in part, characterize the arc-quenching properties of the mixture.
Sulfur hexafluoride is also of great interest to the researchers due to the
fact that ions, such as SF5+, formed in its discharges
are known to be very effective components of etching plasmas. Therefore,
to model and
optimize the properties of such plasmas cross sections for ion-molecule
collisions involving these ions and certain neutrals need to be measured. This
work is planned for the nearest future.
Currently, in cooperation with Johns Hopkins University Applied Physics
Laboratory we participate in development of a miniature plasma
spectrometer for NASA. Our role is to evaluate and test its prototype using
our versatile ion-beam apparatus.
To learn about graduate research opportunities at AMO physics labs,
please contact
Dr. Champion
or visit our laboratories in rooms 321 and 313 of Small Hall (Physics
Department).
Up
[1] B.L. Peko, R.L Champion, Y. Wang, J. Chem. Phys. 104 (1996) 6149.
[2] B.L. Peko, R.L. Champion, J. Chem. Phys. 107 (1997) 1156.
[3] B.L. Peko, I.V. Dyakov, R.L. Champion, J. Chem. Phys. (109) (1998)
5269.
[4] B.L. Peko, I.V. Dyakov, R.L Champion, M.V.V.S. Rao, J.K. Olthoff, Phys.
Rev. E 60 (1999)
7449.
Front page -
Introduction -
Surface -
Gas phase -
Physics Dept.
|